 Promoter : ALTHEYS 42 avenue Julien 63000 Clermont-Fd France
Promoter : ALTHEYS 42 avenue Julien 63000 Clermont-Fd France
Dates of study January 2016 to December 2016
INTRODUCTION :
1.1. Epidemiology of atopic dermatitis
Atopic dermatitis prevalence has increased in 30 years from 5-10% to 10-25%. Over the last decade, epidemiological data have been more numerous and not focused on respiratory atopic manifestations only. Most authors place a premium on environmental changes during early childhood to explain this increase. Atopic dermatitis is a chronic pruriginous skin disease evolving by relapses. Genetics have highlighted a major causal element for atopic dermatitis, which places the barrier of cutaneous permeability at the center of its physiopathology. The multiple causes cited for relapses in published studies are difficult to identify with certainty. Some causes, such as external temperature, irritants and aero-allergens have an already epidemiologically or experimentally demonstrated impact on this barrier anomaly.
1.2. Treatments of atopic dermatitis
The goal of treatments is to suppress symptoms as much as possible and to control eczema outbreaks in the long term. It is only after the inflammation / eczema has been cured that the epidermal barrier can be maintained optimally intact by means of basic local moisturizers. Most treatments are based on the following principles :
- restoration of the epidermal barrier by a suitable topical product (skin cleansing and restorative care).
- prophylaxis and control of skin infections by means of regular skin cleansing and topical anti-inflammatory treatments (antiseptics, if necessary antibiotics and corticosteroids).
- anti-inflammatory treatments with the goal of quickly curing eczema outbreaks and prevent recurrence.
- restoration of the epidermal barrier, which plays a preponderant role in the treatment is done with non-medicated emollient products, whose formula includes moisturizers and lipids to act as substitutes for sebum whose secretion is impaired.
1.3. Evaluation of atopic dermatitis
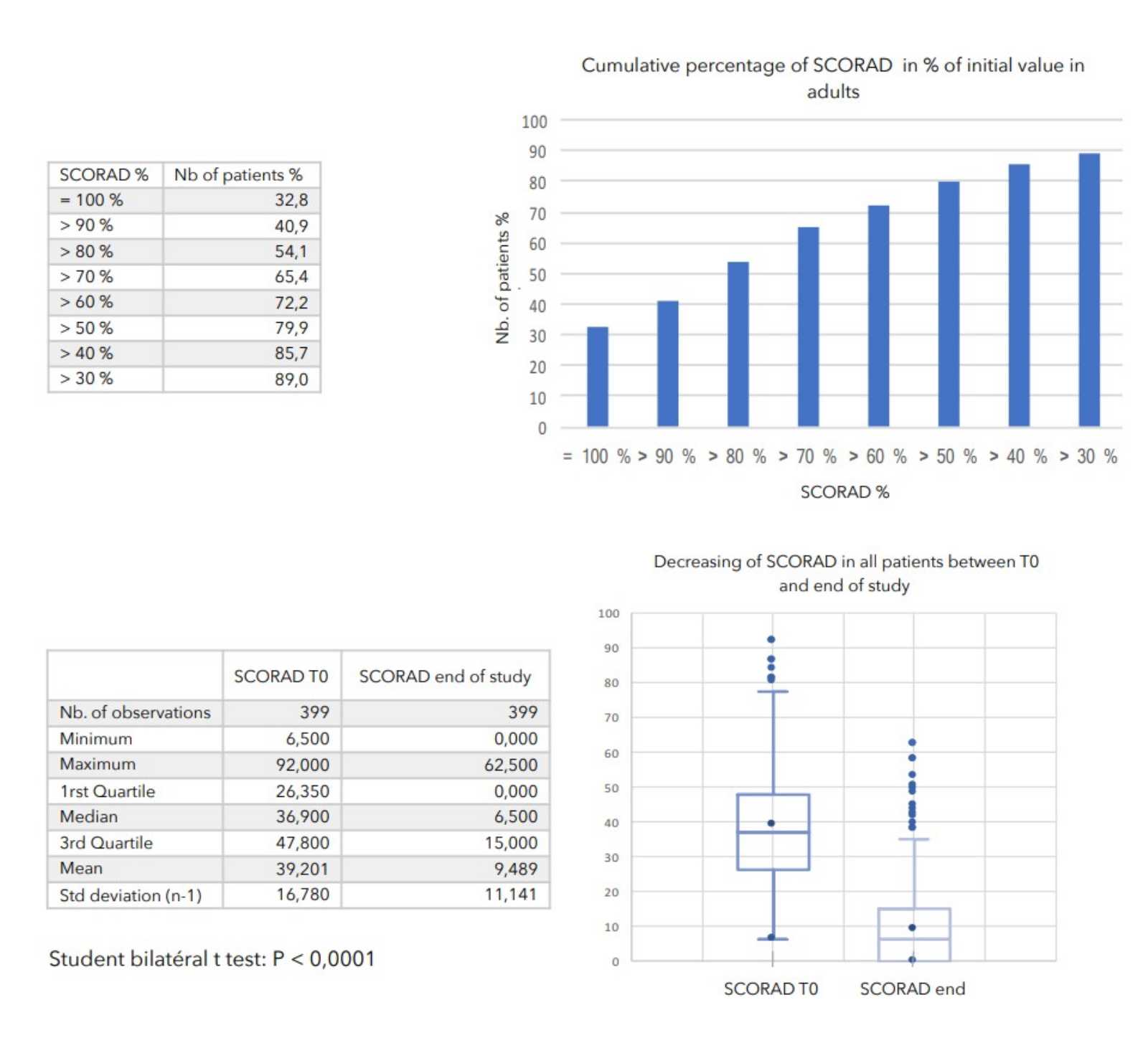
SCORAD (Scoring atopic dermatitis) is the severity score of atopic dermatitis. It was created and validated in 1990 by a group of experts: the European Task Force of Atopic Dermatitis. SCORAD has become a reference tool for the monitoring and evaluation of pathology by physicians in Europe. SCORAD exists in the form of a paper or digitalised form which the physician fills during a consultation in order to obtain a score after the relevant calculation. It thus makes it possible to follow the evolution of the patient’s atopic dermatitis.
2. NATURE OF THE COHORT STUDY
The study was a longitudinal and non-interventional observatory, dealing with patients with atopic dermatitis, on the evolution of their SCORAD following the application of Zematopic® eczema therapy body cream and on its tolerance.
Zematopic® eczema therapy body cream has the regulatory status of a medical device and is therefore not a medicine. This observatory was carried out according to the doctor's usual medical practice and did not impose any additional diagnostic or therapeutic procedure.
This study was non-interventional.
3. GOALS OF THE STUDY
Following in "real life" for 1 month, a cohort of patients with atopic dermatitis, and receiving Zematopic® eczema therapy body cream as additional treatment.
The primary objective of the study was to evaluate the evolution of patients SCORAD.
The secondary objectives of the study were:
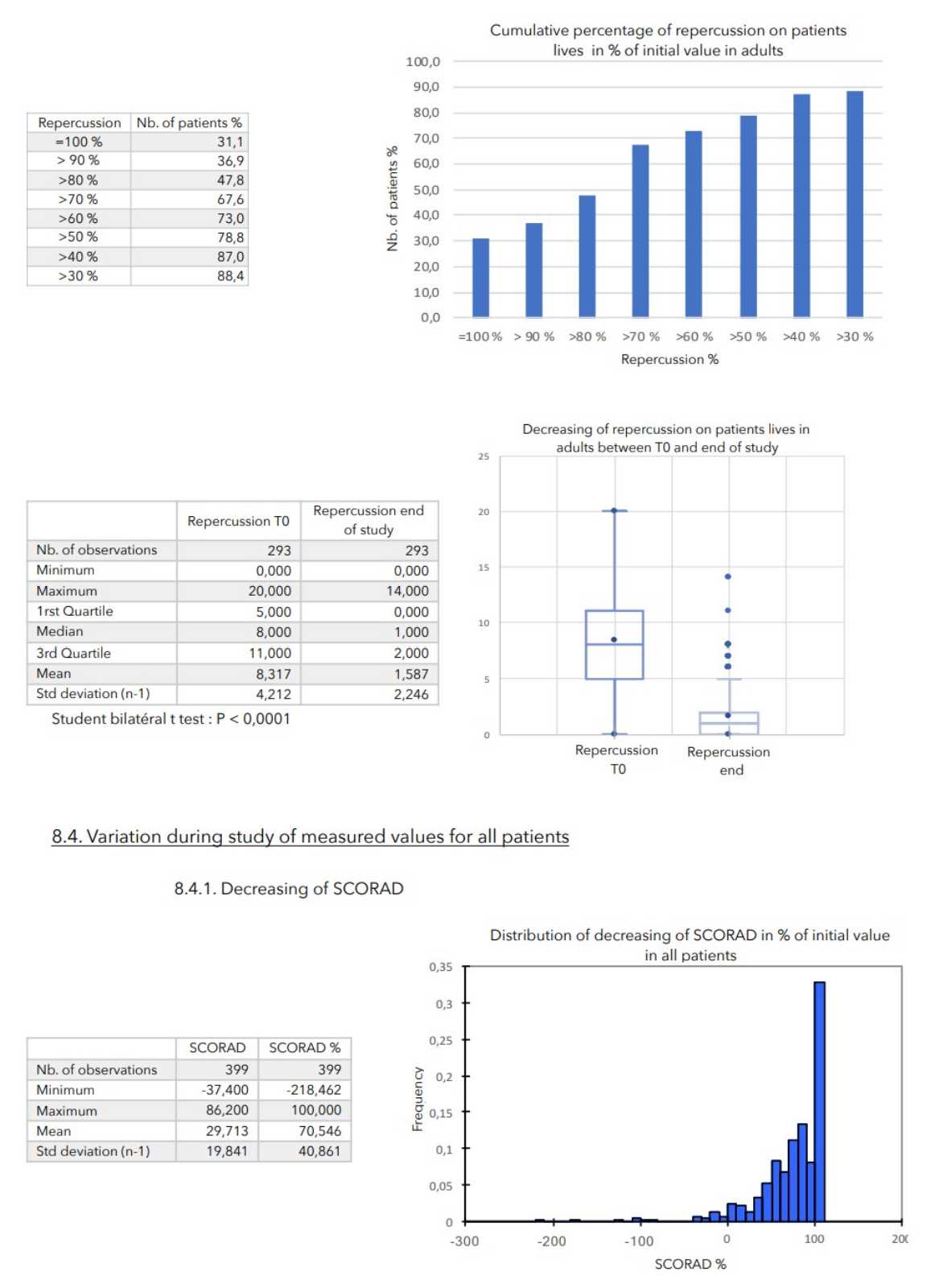
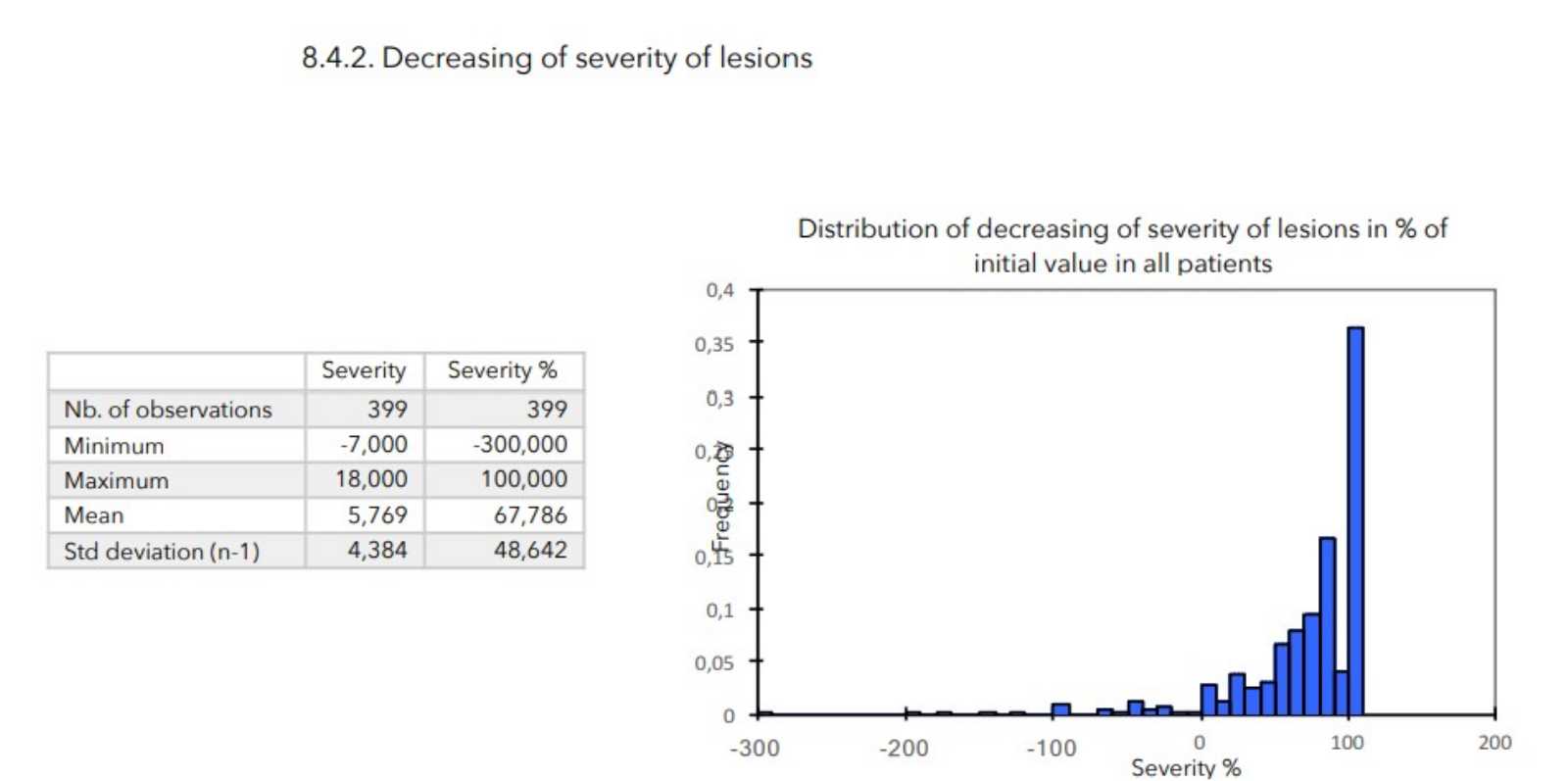
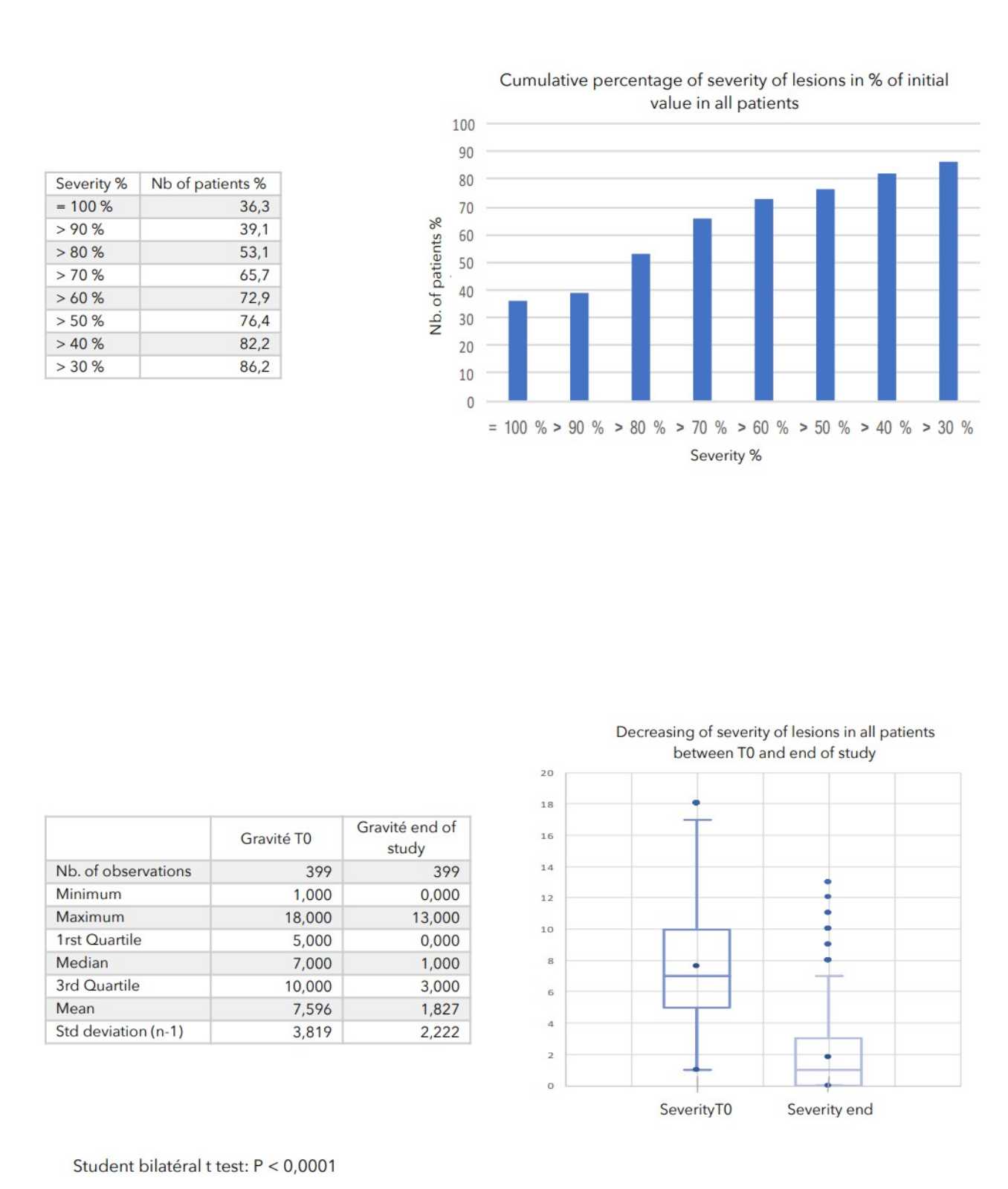
- evaluate the tolerance of Zematopic® eczema therapy body cream during this study, ALTHEYS cohort study page 2 on 23 - evaluate the evolution of the severity of the lesions as well as the disease’s repercussion on patients' lives.
4. LEGAL PROVISIONS
4.1. French law of 6 January 1978, modified relating to data, files and freedoms
Patient data were collected indirectly nominatively. No patient name has been reported on the study documents, the only code allowing the input control was a code corresponding to the inclusion number.
The computer processing of patient data and doctors’ files, done within the framework of this observatory, authorized by the National Commission of the Computing and Freedoms (CNIL) after opinion of the Consultative Committee on the Treatment of the Information in the field of Health Research (CCTIRS).
4.2. Committee for the Protection of Persons
This observatory did not include any guidelines concerning the care of patients. It has not changed the doctor-patient relationship and has therefore not entered into the framework of the law on biomedical research (Law 88-1138 of 20 December 1988 as amended). Therefore, no written consent was requested from patients and a prior request to a PPC for an opinion was not necessary. A simple information note was given to each included patient only.
5. PROGRESS OF THE OBSERVATORY
This observatory was carried out in France and took place from January to December 2016.
5.1. Implementation of the observatory
At the setting up of the observatory, each doctor received a set-up kit consisting of:
- inclusion sheets for follow-up and discharge of her patients,
- the study protocol,- a guide for calculating SCORAD,
- administrative elements.
At the first consultation, the doctor informed the patient of the carrying out of the observatory. She fills the inclusion sheet and the patient is given a letter of information about the observatory.
5.2. Patient following
The doctor followed the patient according to his usual practice.
At the end of each patient follow-up visit, the physician completed a follow-up sheet on the clinical data and SCORAD calculation.
5.3. End of the observatory
The study lasted 29 days on average.
For all patients at the end of about 4 weeks follow-up, the doctor filled all the inclusion and end cards for each patient.
5.4. Monitoring the observatory
- Centralized telephone monitoring was provided by ALTHEYS
- Follow-up of the doctors : answering their questions, logistic follow-up and regular filling and return of the documents according to the pre-established calendar, and following medical requests on incomplete data.
- An on-site visit, planned for all participating physicians, during the observatory’s setting up.
In addition, a hotline provided by ALTHEYS was made available to participating physicians throughout the observatory to answer requests for information on the implementation and conduct of the study.
5.5. Data collection
Patient information was collected via inclusion, follow-up and exit cards as non-identifying data.
6. MATERIAL AND METHODS
6.1. Protocol
6.1.1. Participating physicians
31 general practitioners and liberal specialists participated in this observatory.
6.1.2. Selected patients
The selected patients were patients of the participating physicians. They were included in the observatory consecutively as they went consulting.
6.1.3. Criteria for inclusion of patients
- Patient suffering from atopic dermatitis for which their doctor had planned to add Zematopic® eczema therapy body cream to their possible treatment.
6.1.4. Criteria for non-inclusion of patients
- Patient with another concomitant dermatological disease.
- Patient participating, or having participated a biomedical research the preceding month.
- Patient with an allergy to one of the product’s constituents
- Patient who refused to participate in the study.
6.1.5. Data collected
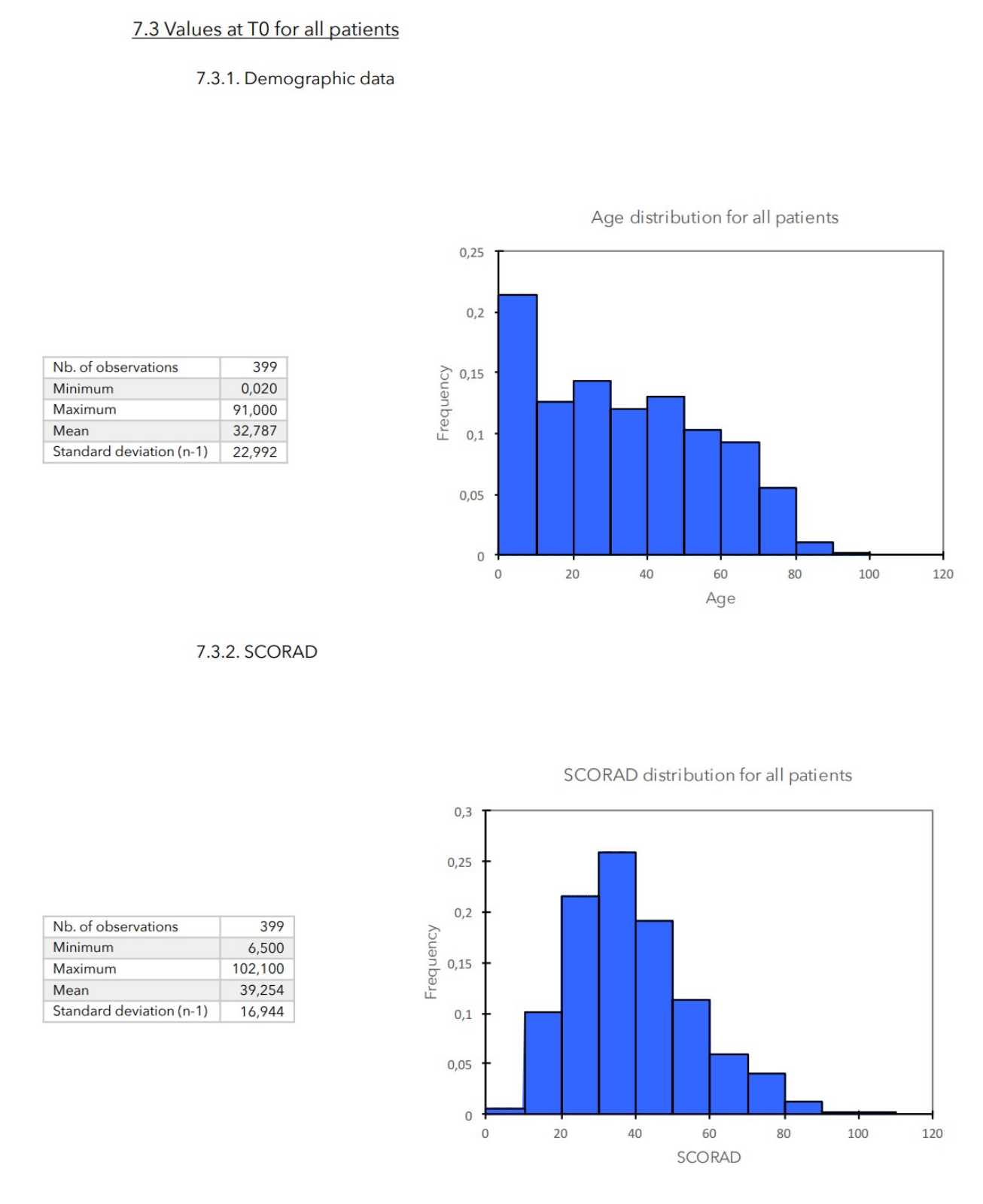
T0: - General clinical data of the patient (age, weight, sex).
- Data relating to the possible medical treatment followed.
- Calculation of SCORAD.
- Application of Zematopic® eczema therapy body cream and collection of the patient's assessment or a patient’s relative's assessment if the patient is a child (score 1 to 10).
T1 = about four weeks:
- Calculation of SCORAD.
- Data relating to the possible medical treatment.
- Continued use of Zematopic® eczema therapy body cream the previous consultation: if stopped or interruption, providing the cause.
- Tolerance: adverse events (number, nature, severity, …).
6.2. Product used
The product used for the study was Zematopic® eczema therapy body cream for the body sold in pharmacies: medical device in white "airless" of 200 ml pump bottles.
6.3. Statistics
6.3.1. Variables
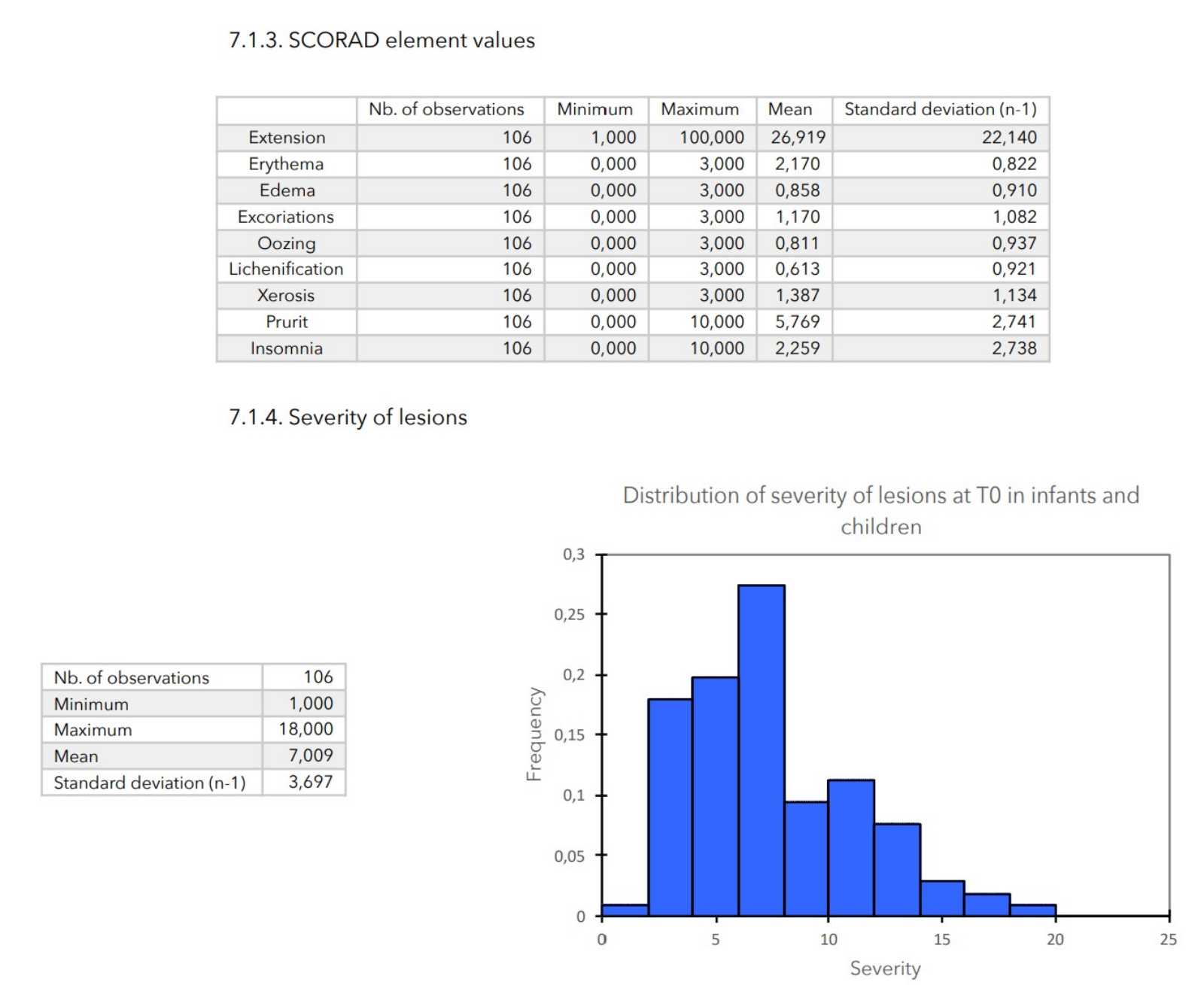
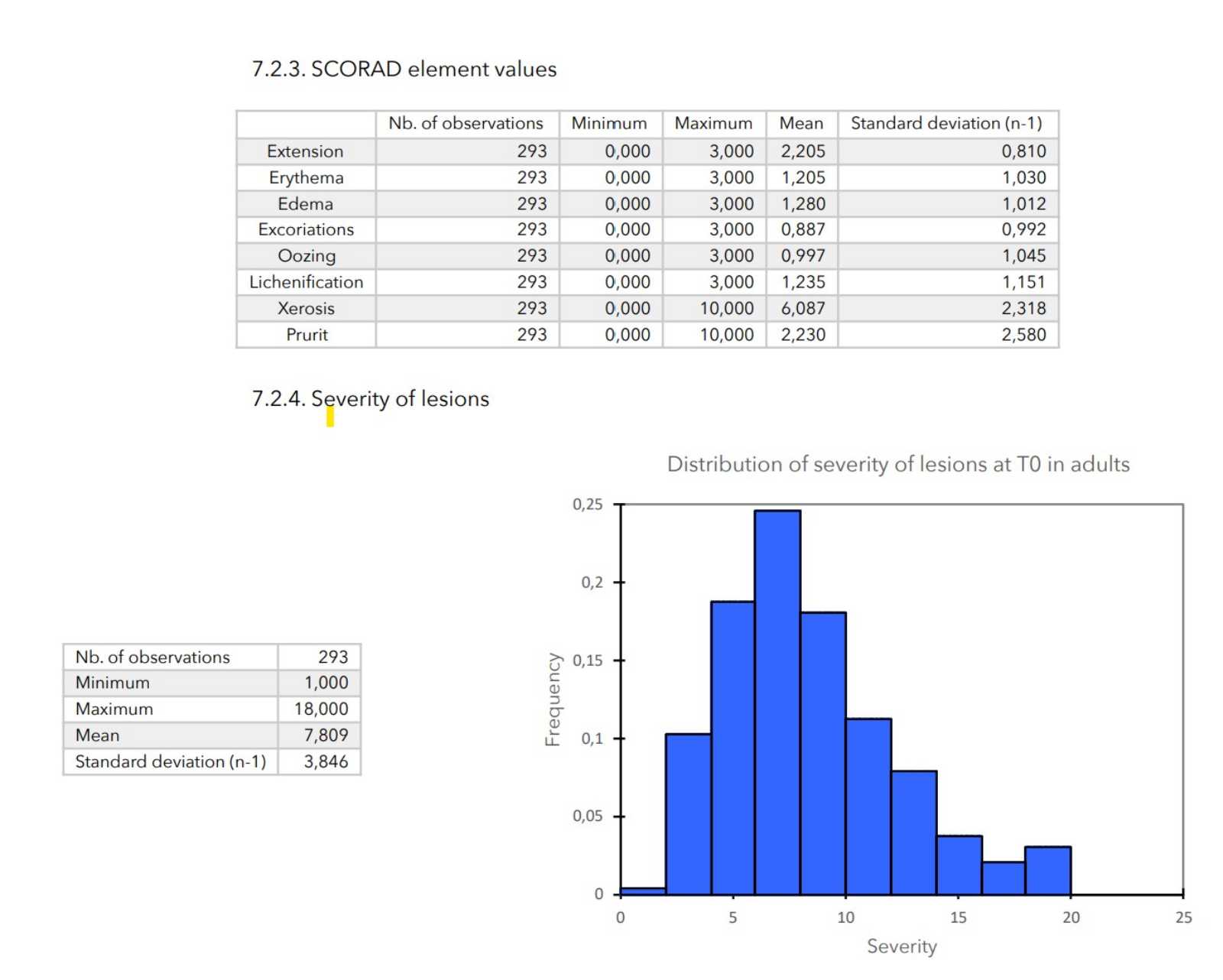
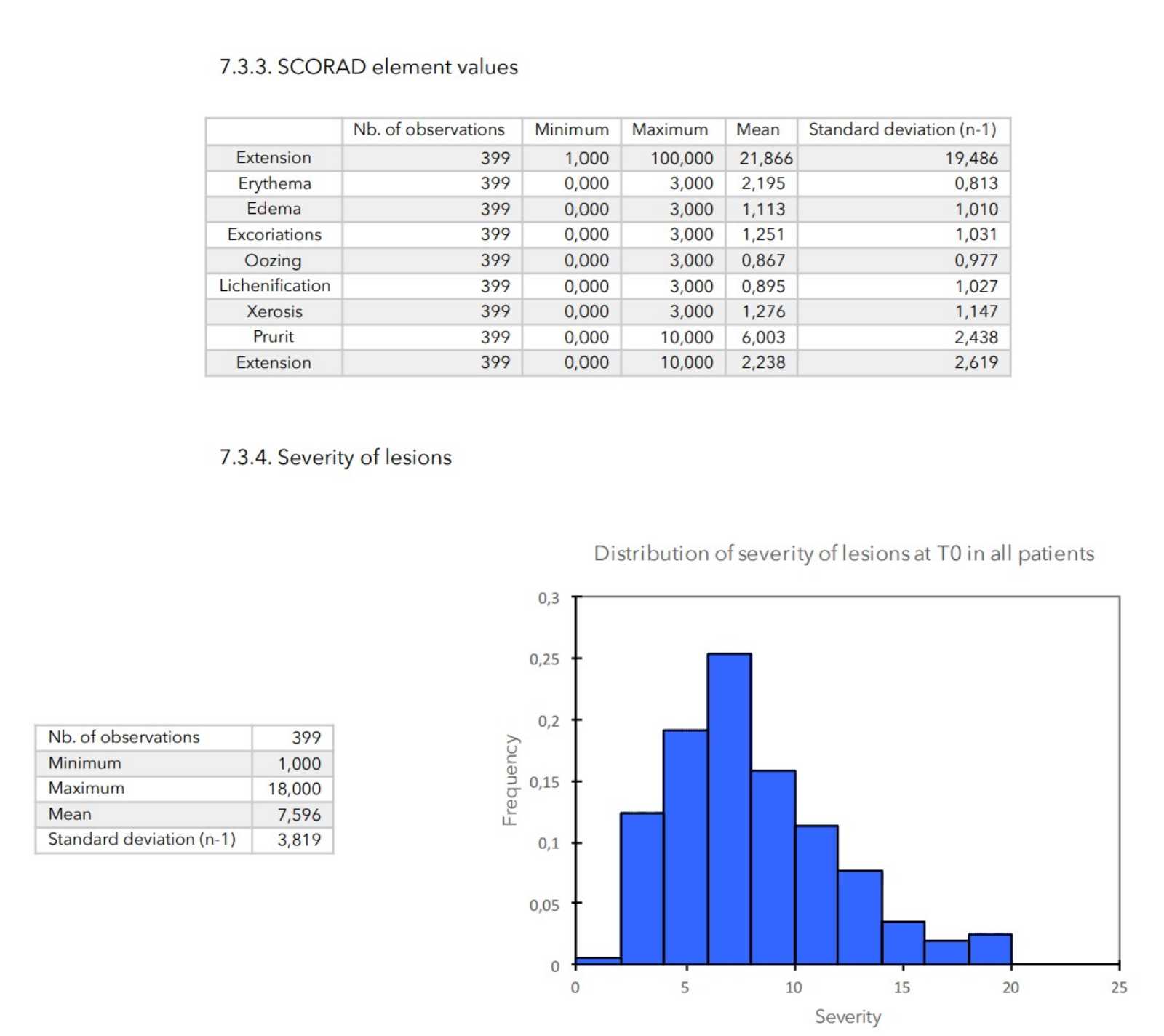
- Elements of SCORAD: scores of 0 to 3 for cutaneous evaluation levels (erythema, edema, excoriation, oozing, lichenification, xerosis), scores of 0 to 10 for pruritus and insomnia and score from 0 to 20 for the general impact of the disease on the patient (calculated as the sum of the pruritus and insomnia scores).
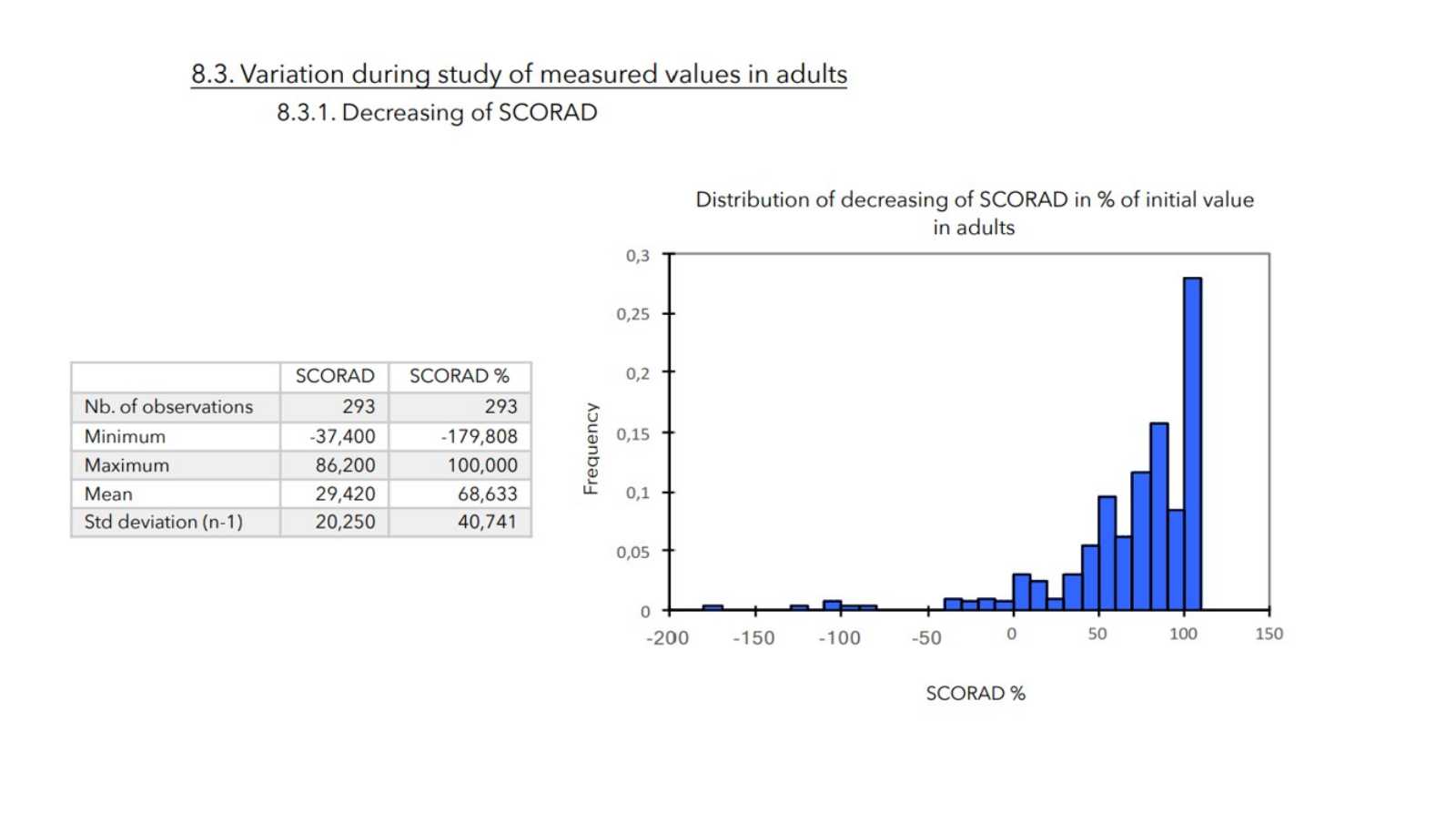
- SCORAD is calculated as follows:
SCORAD = (Extension / 5) + (7 (Severity / 2) + Sounding.
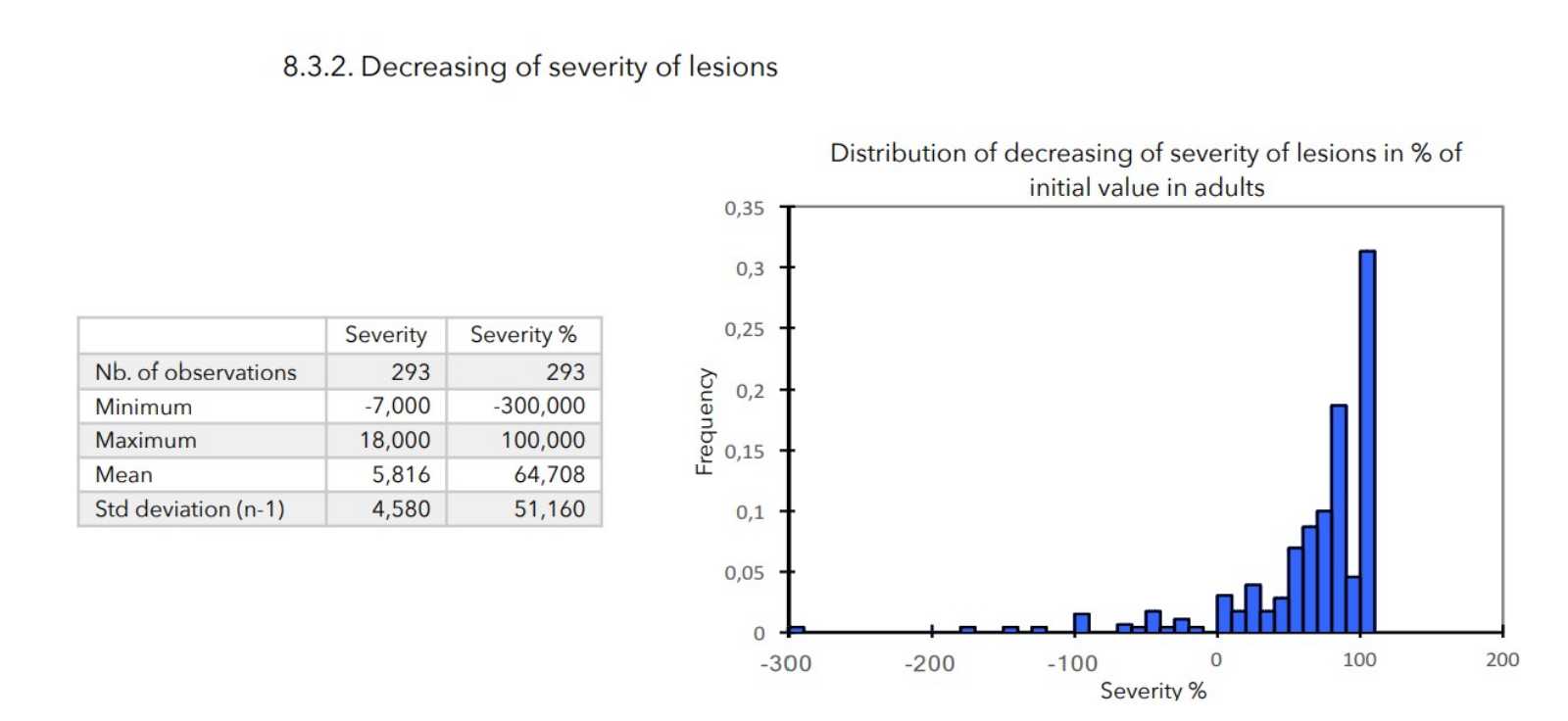
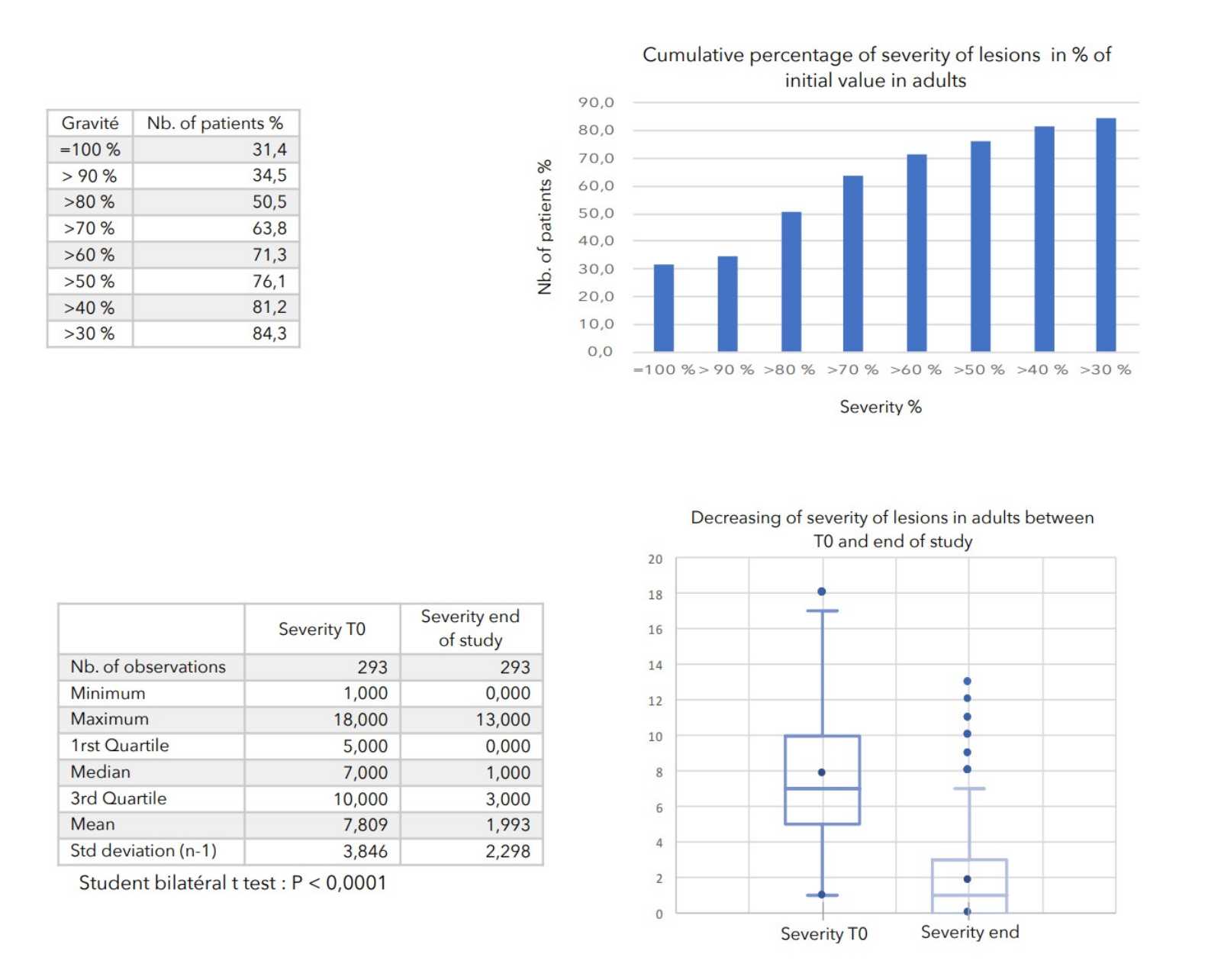
- Severity of lesions: calculated as the sum of the cutaneous evaluation scores.
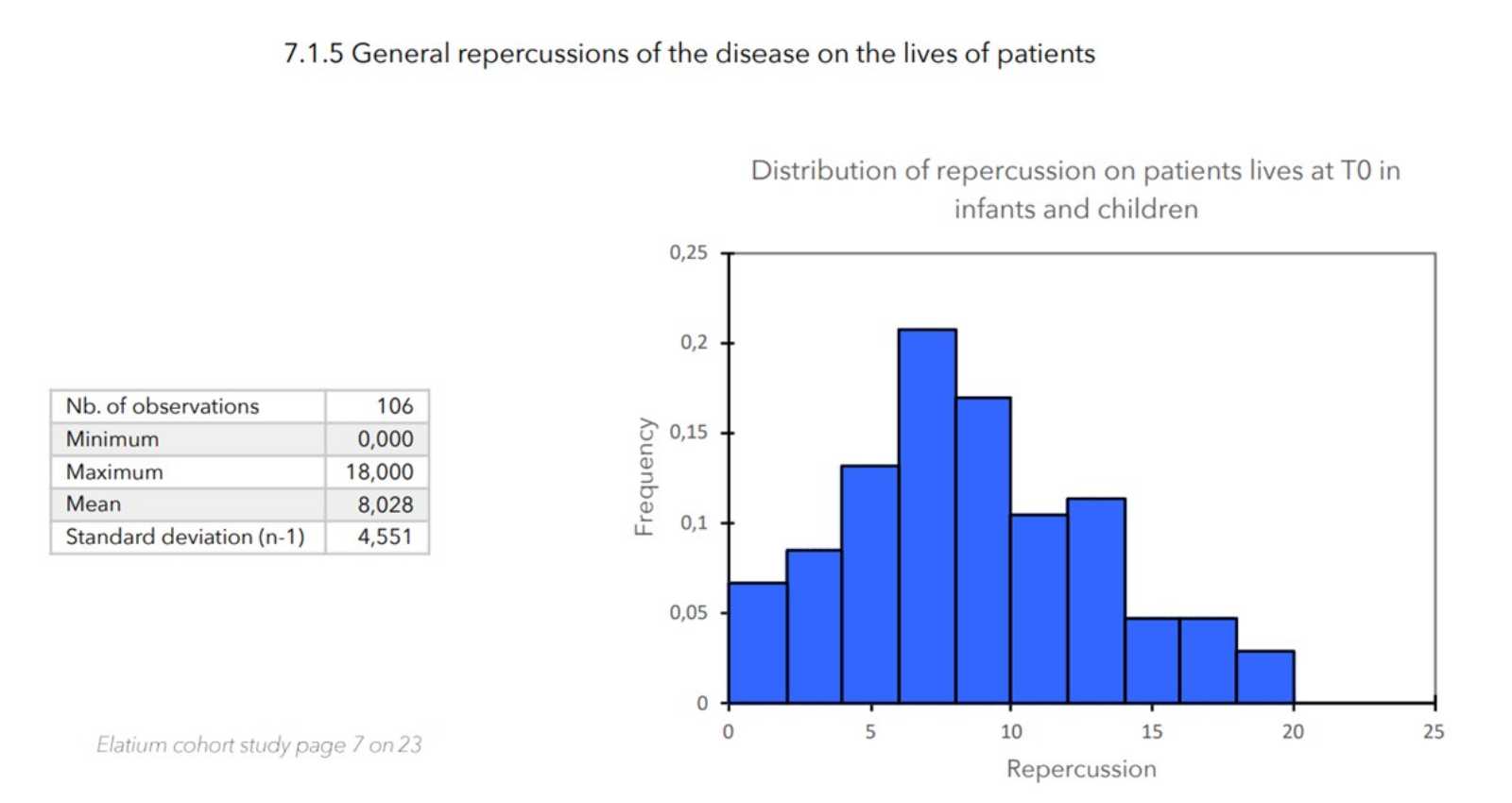
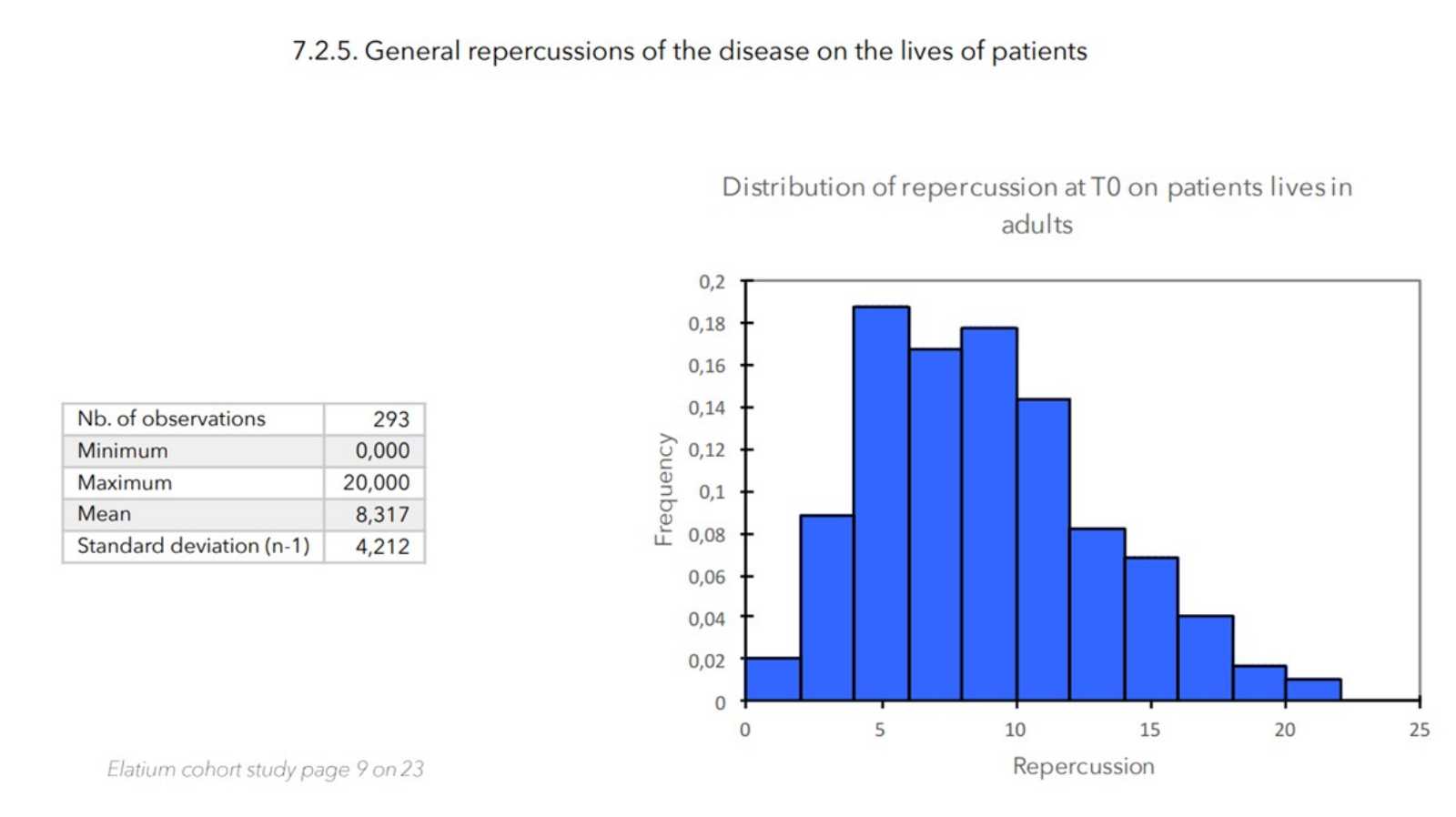
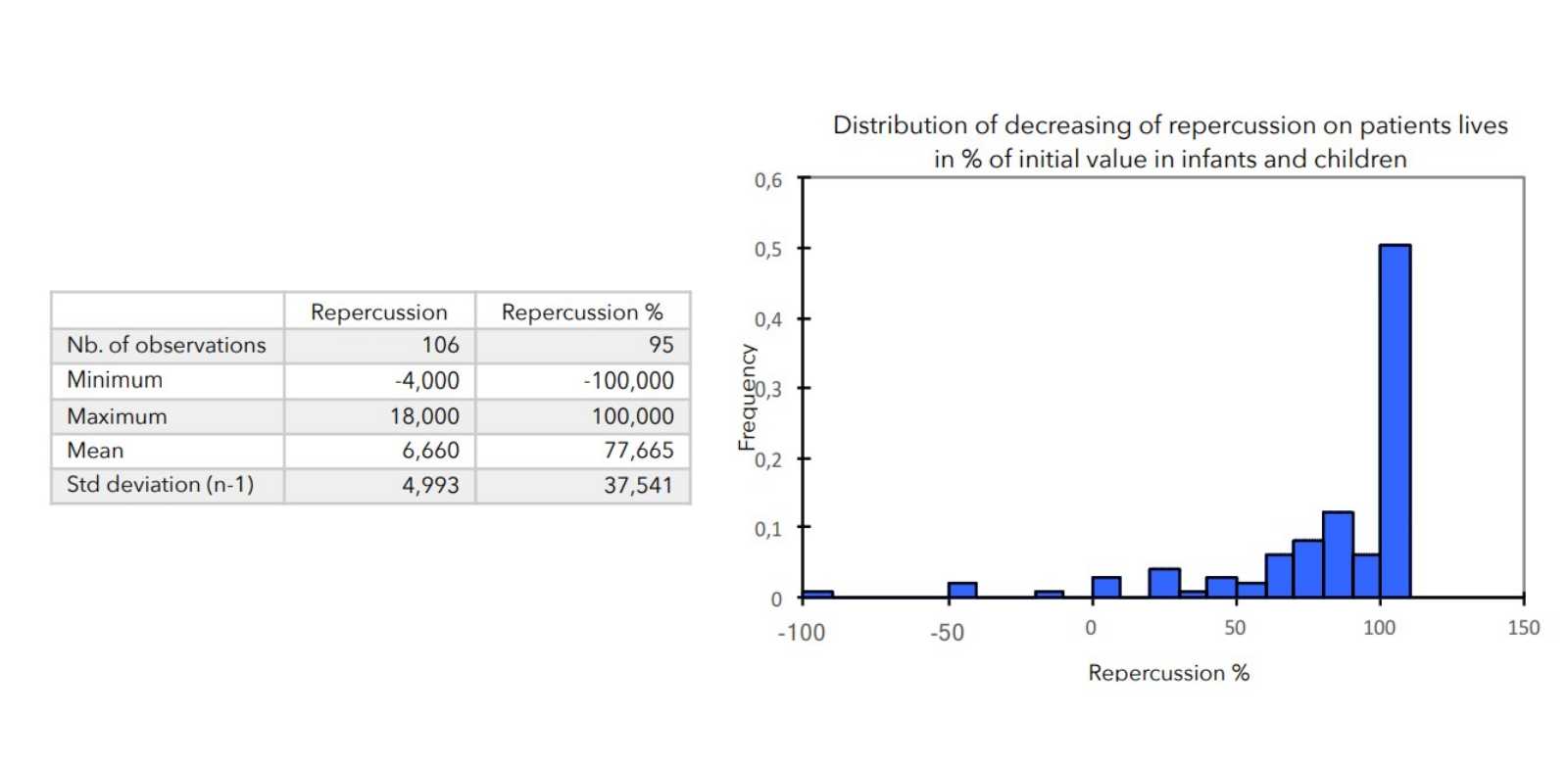
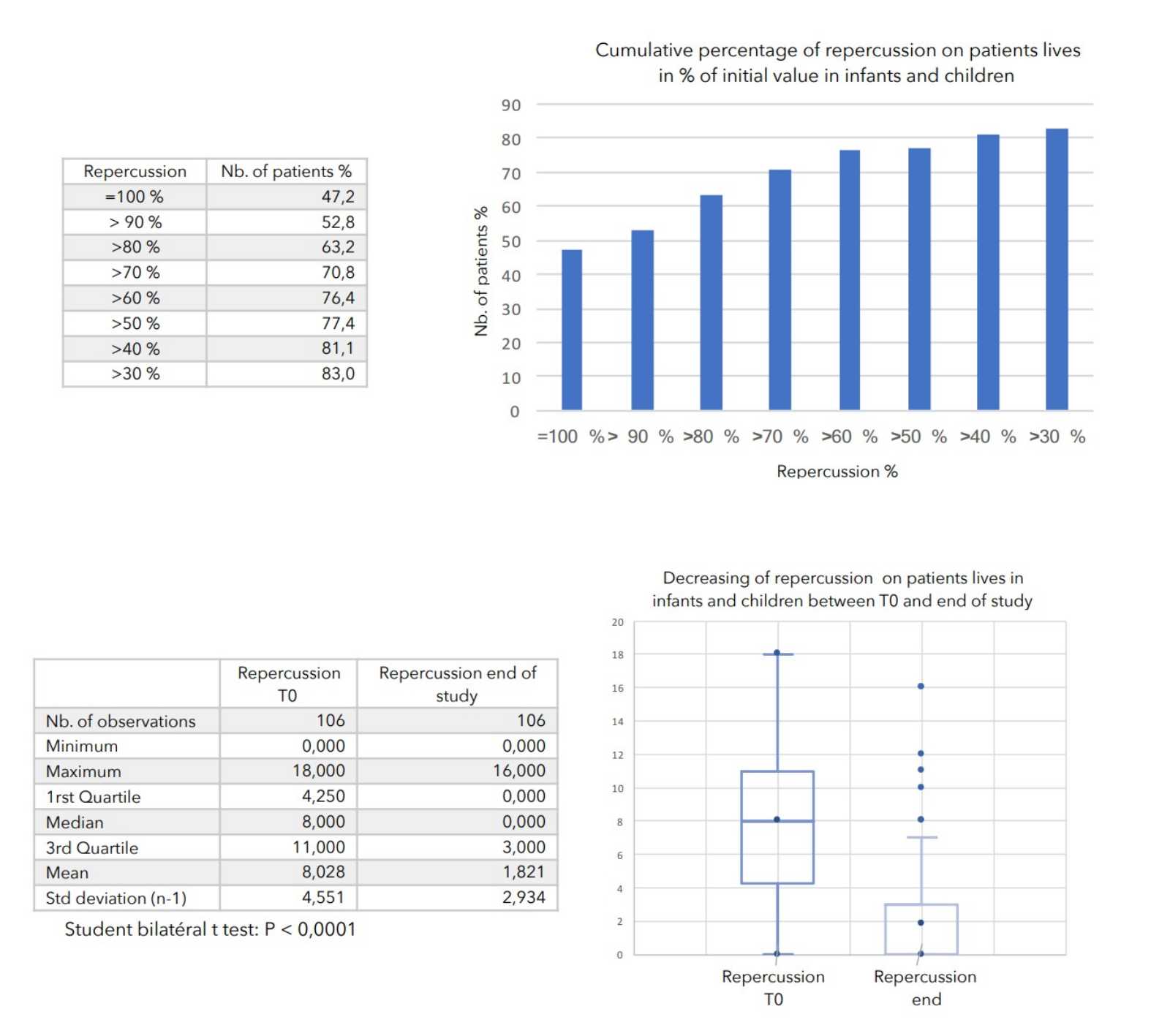
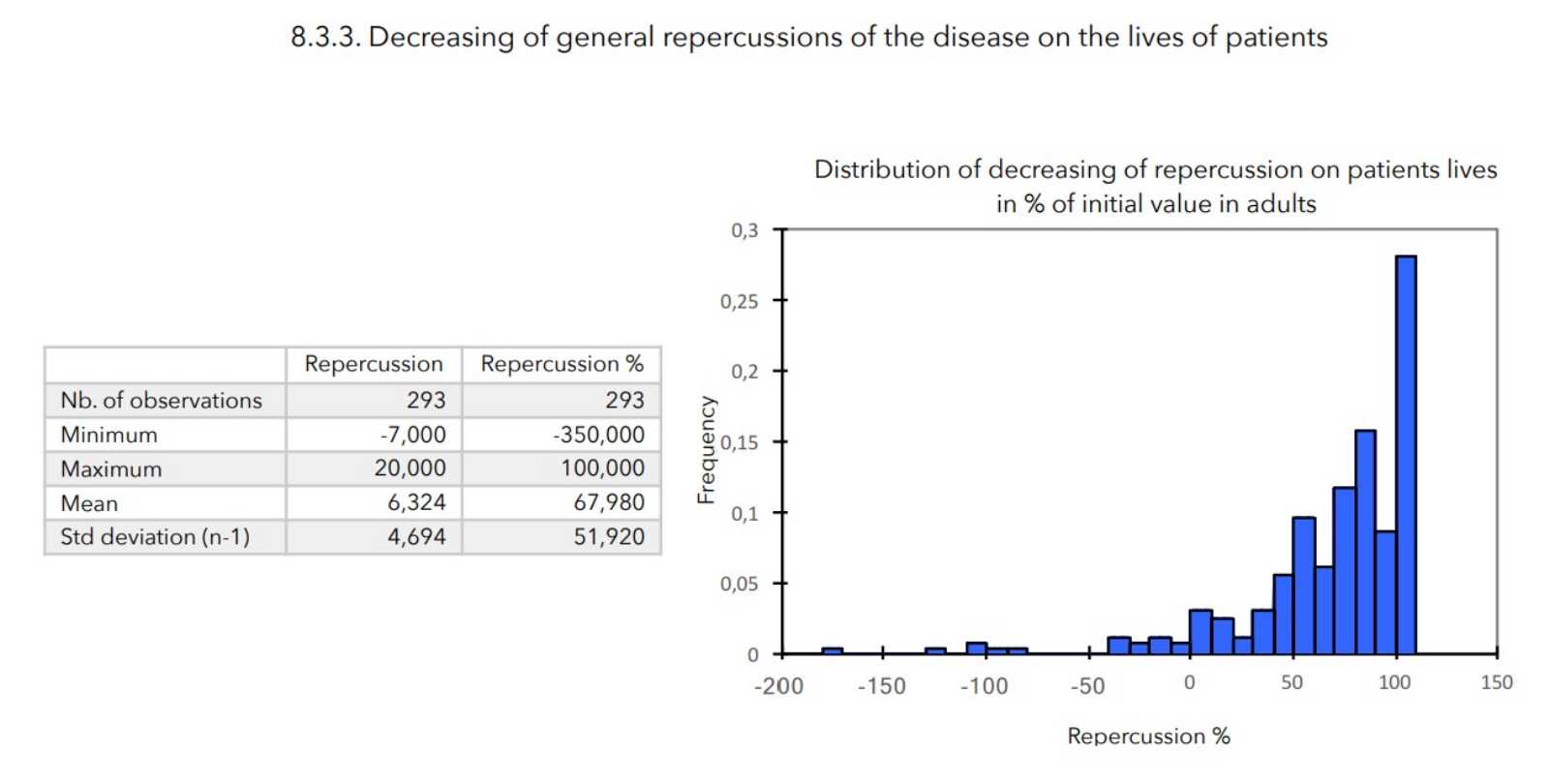
- Value of the general repercussion of the disease on the patient’s life:
Pruritus score + insomnia score -
Undesirable effects (frequency, nature, causality and severity).
6.3.2. Population of analysisThe analysis of primary and secondary variables was performed on the included patient population at each follow-up time.
6.3.3. Outliers
Outliers are defined as any data beyond the outer limits of the data distribution (interquartile calculation). Outliers, after clarification to the physician concerned, if they were not the result of an obvious manifest error, were included in the statistical calculations.
6.3.4. Statistical analysis and evaluation of results
The results of the collected data’s descriptive analysis are presented classically:
-for quantitative variables, by the means plus or minus one standard deviation, the medians and the extreme values, after checking the normality of their distribution
- for qualitative variables, by numbers and percentages. The comparative analysis of the criteria will be made by the Student bilateral t test for gaussian quantitative variables.
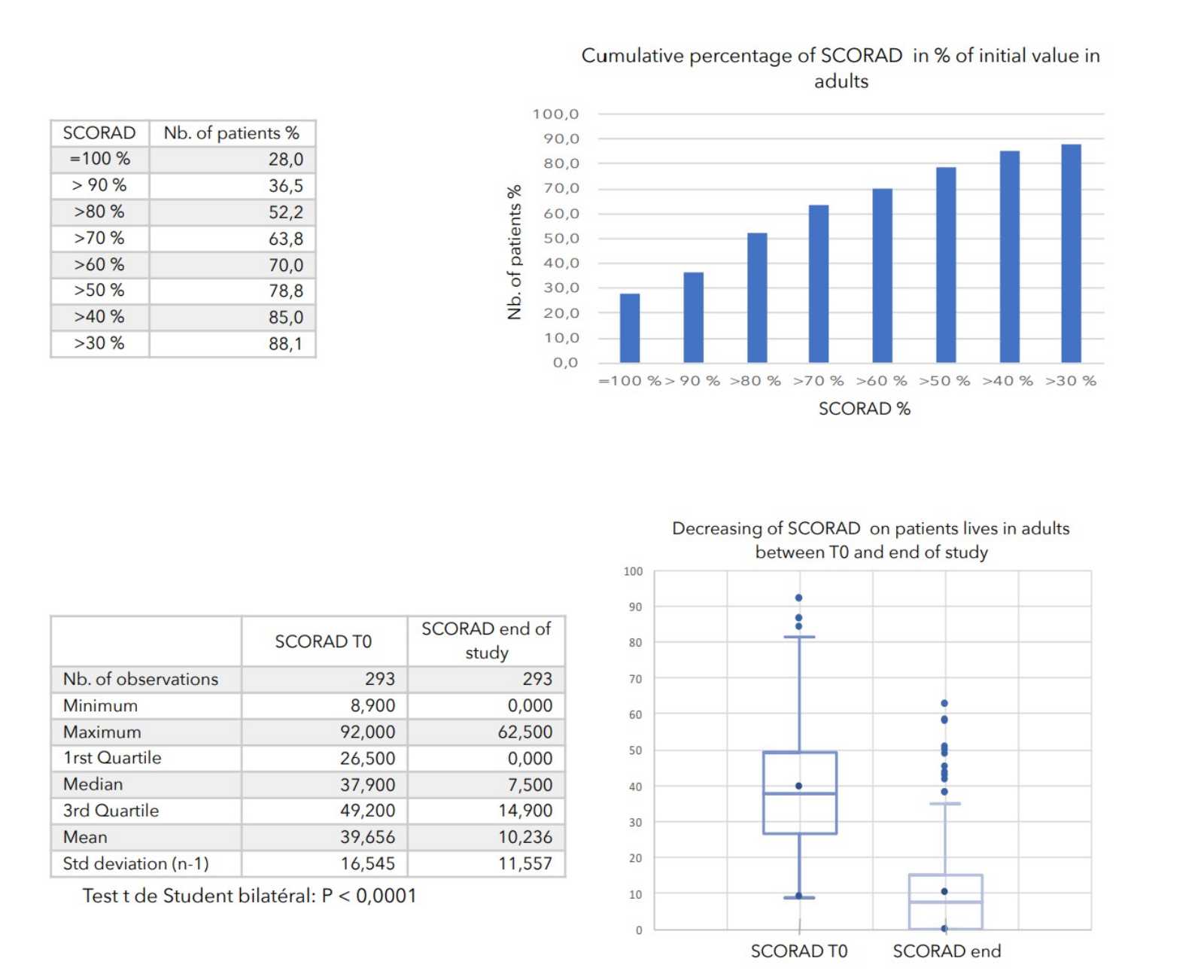
7. VALUES MEASURED AT THE ENTRANCE OF STUDY
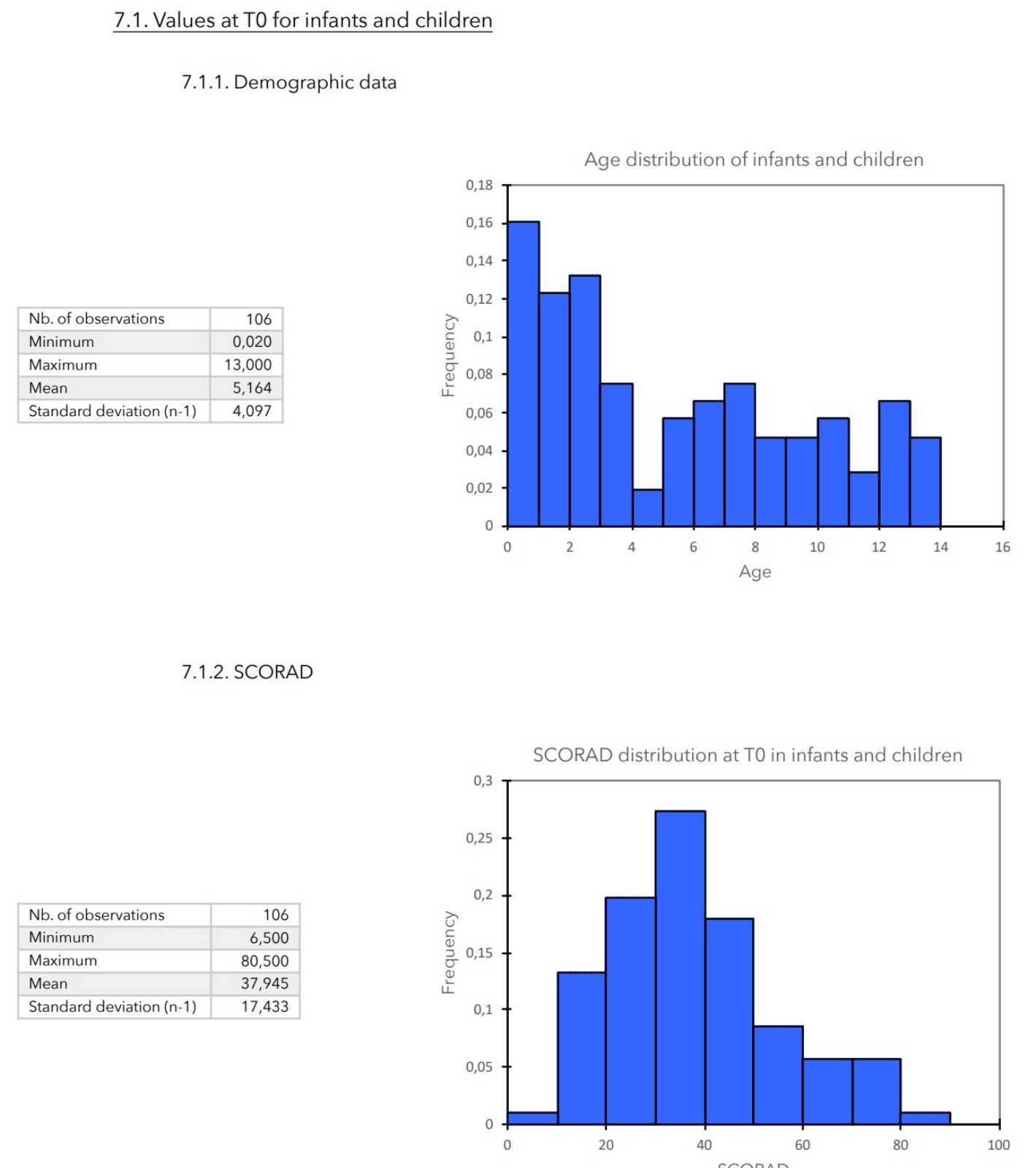
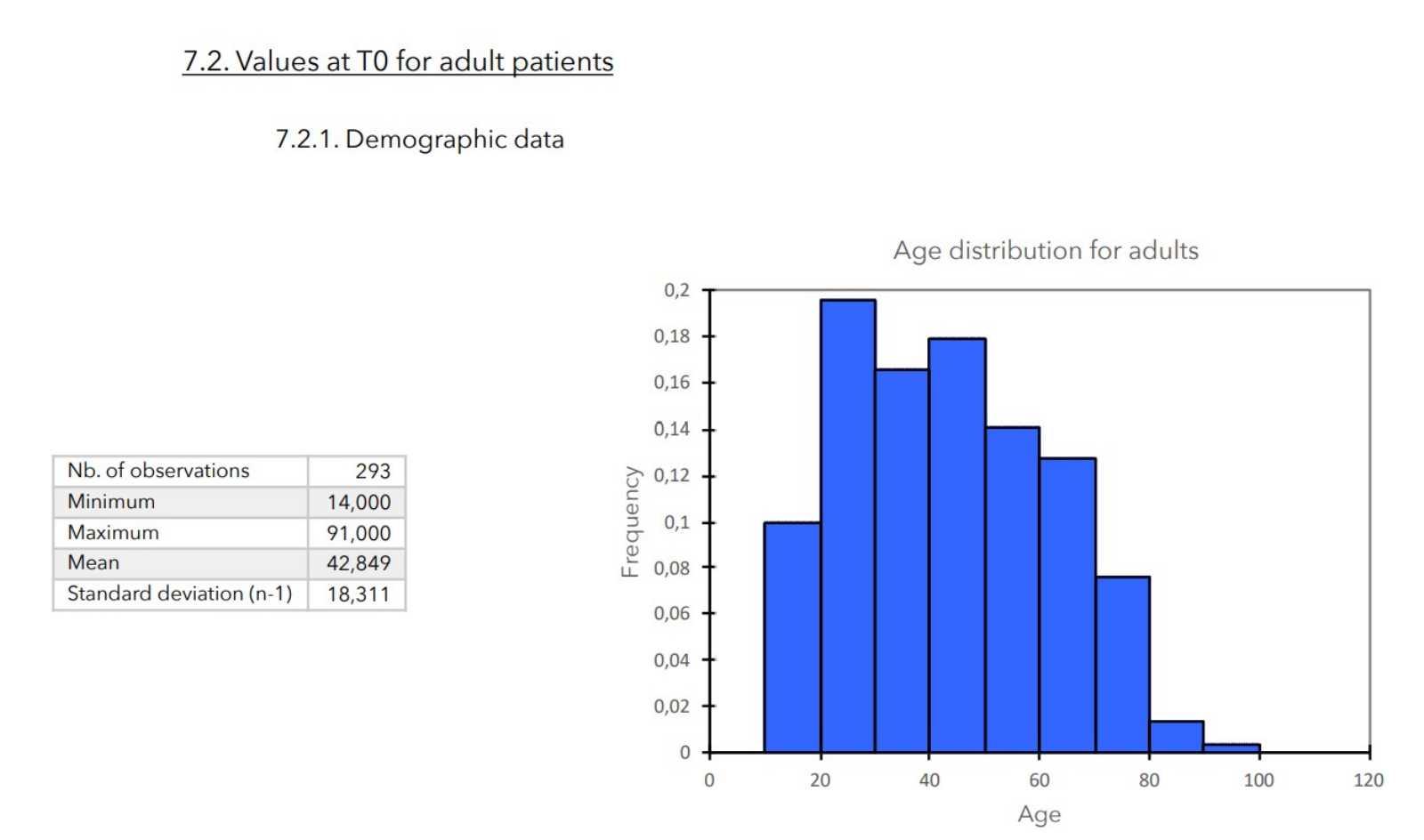
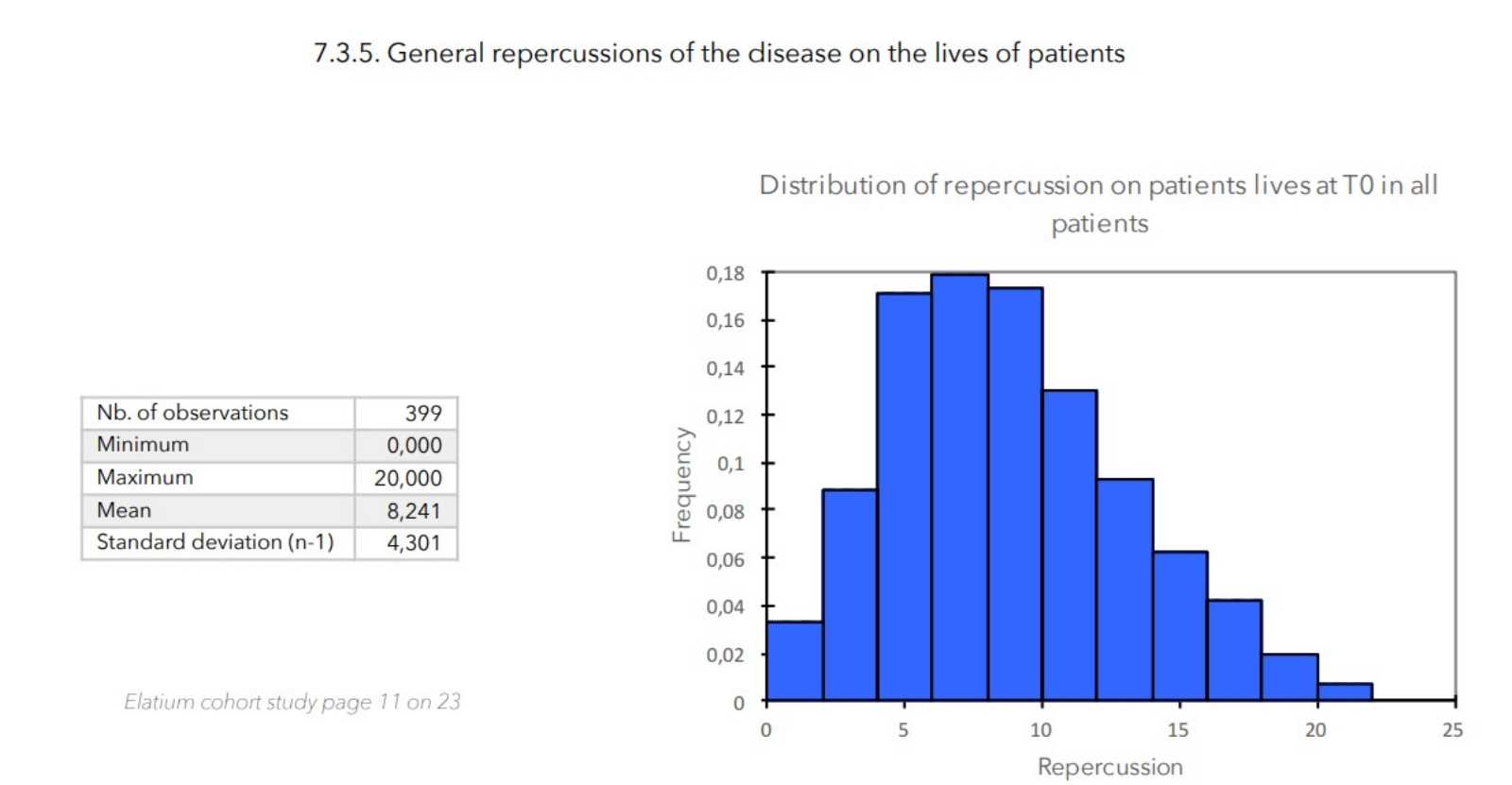
RESULTS
8.1. Adverse effects
No real adverse effects were identified during the study.
At the cream application:
- 2 tingling impressions
- 1 burning sensation
- 1 itching feeling
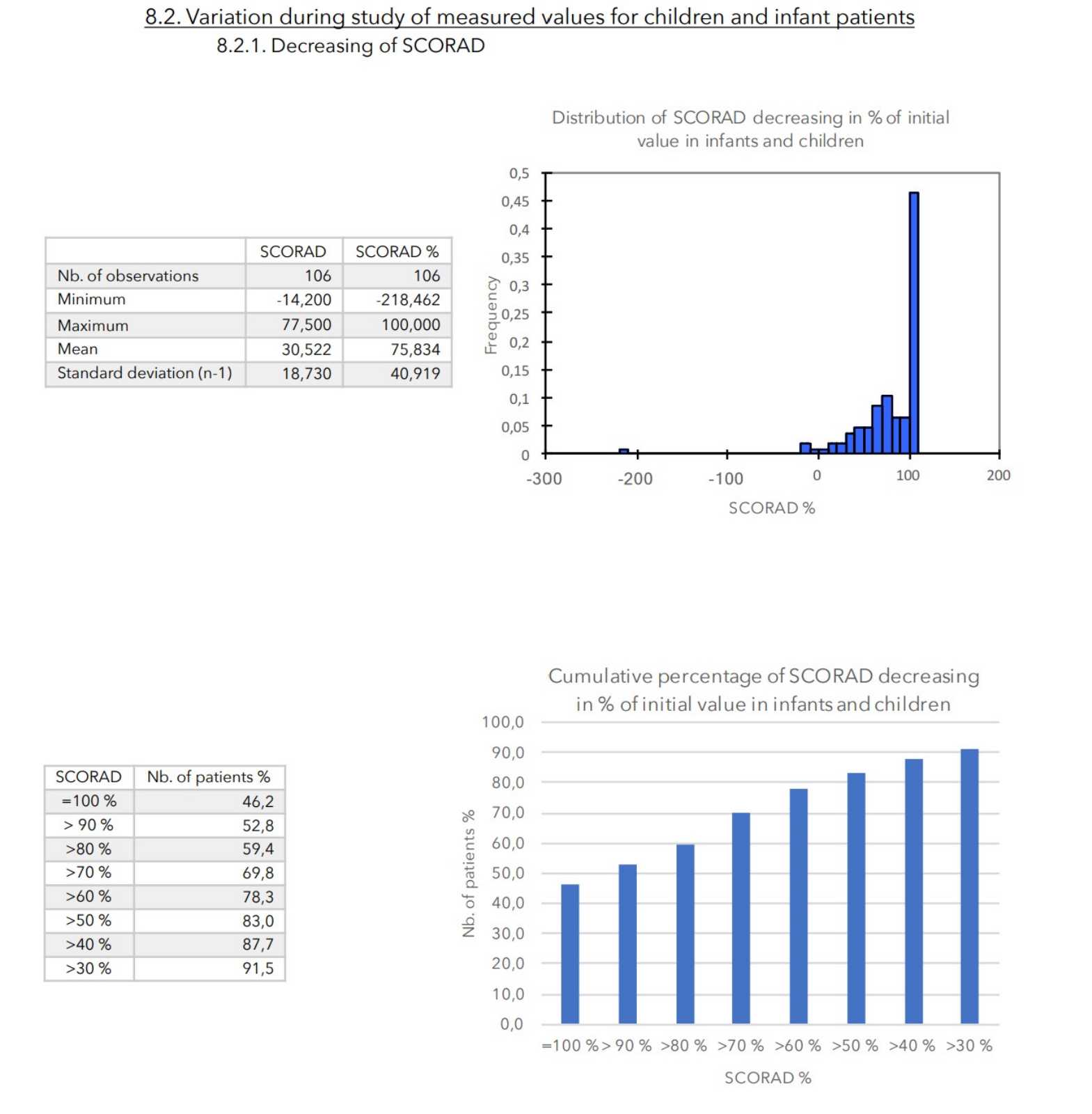
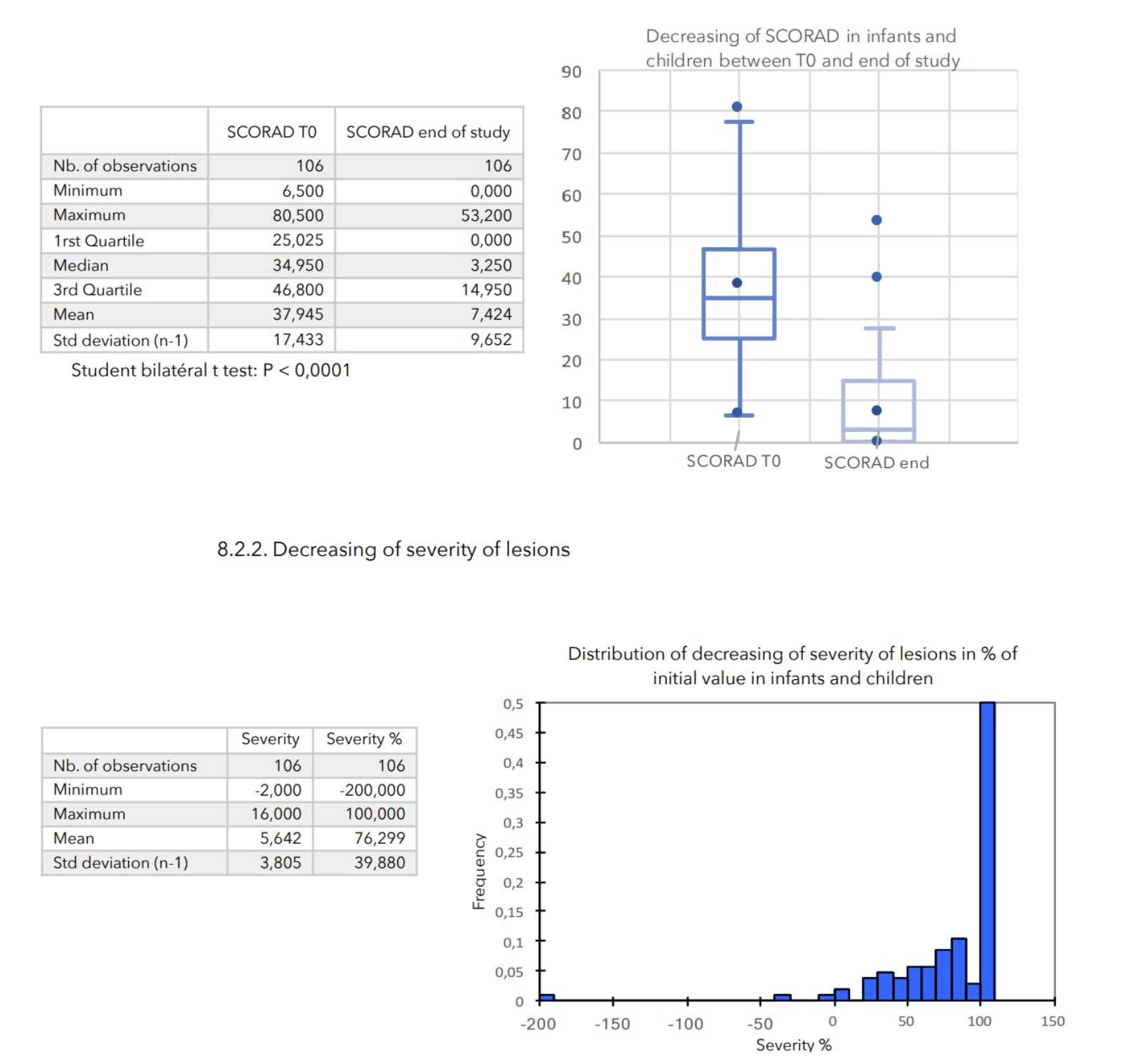
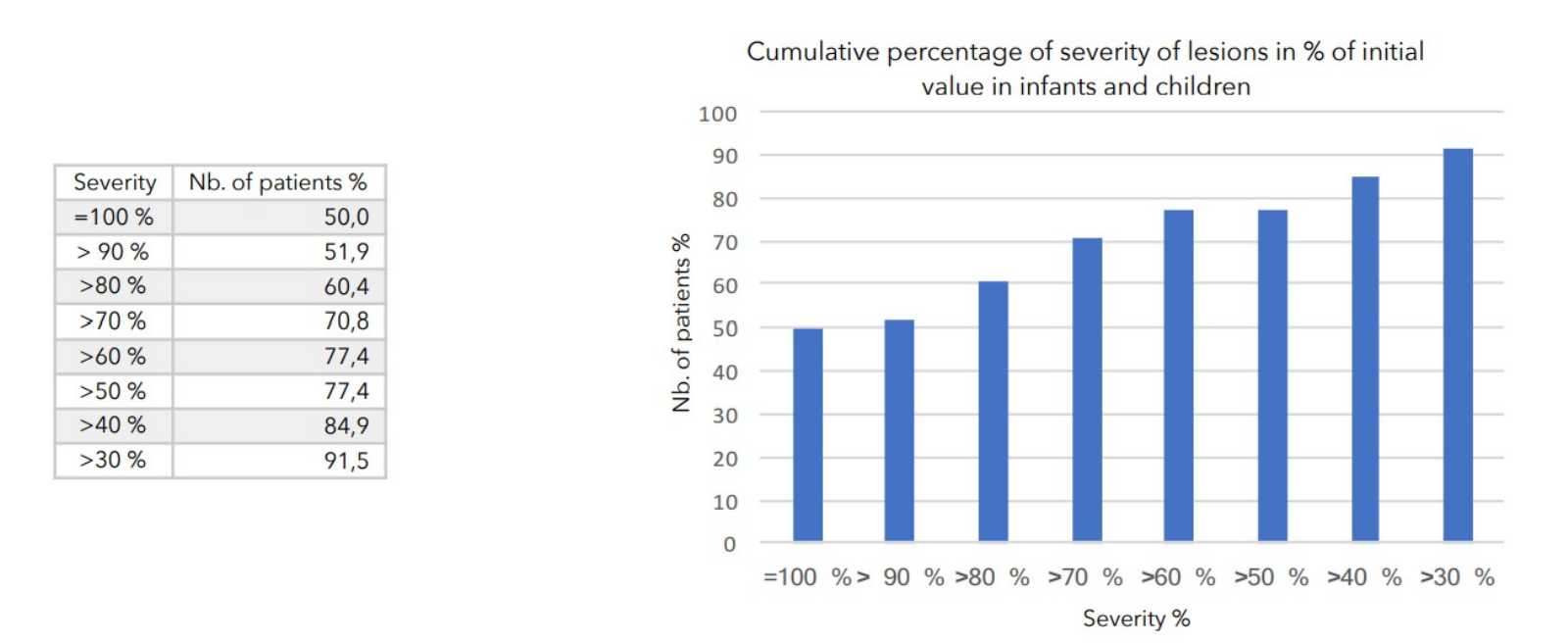
CONCLUSIONS:
During an average of 4 weeks treatment, no truly adverse effects were noted in any of the 399 patients included in the study.
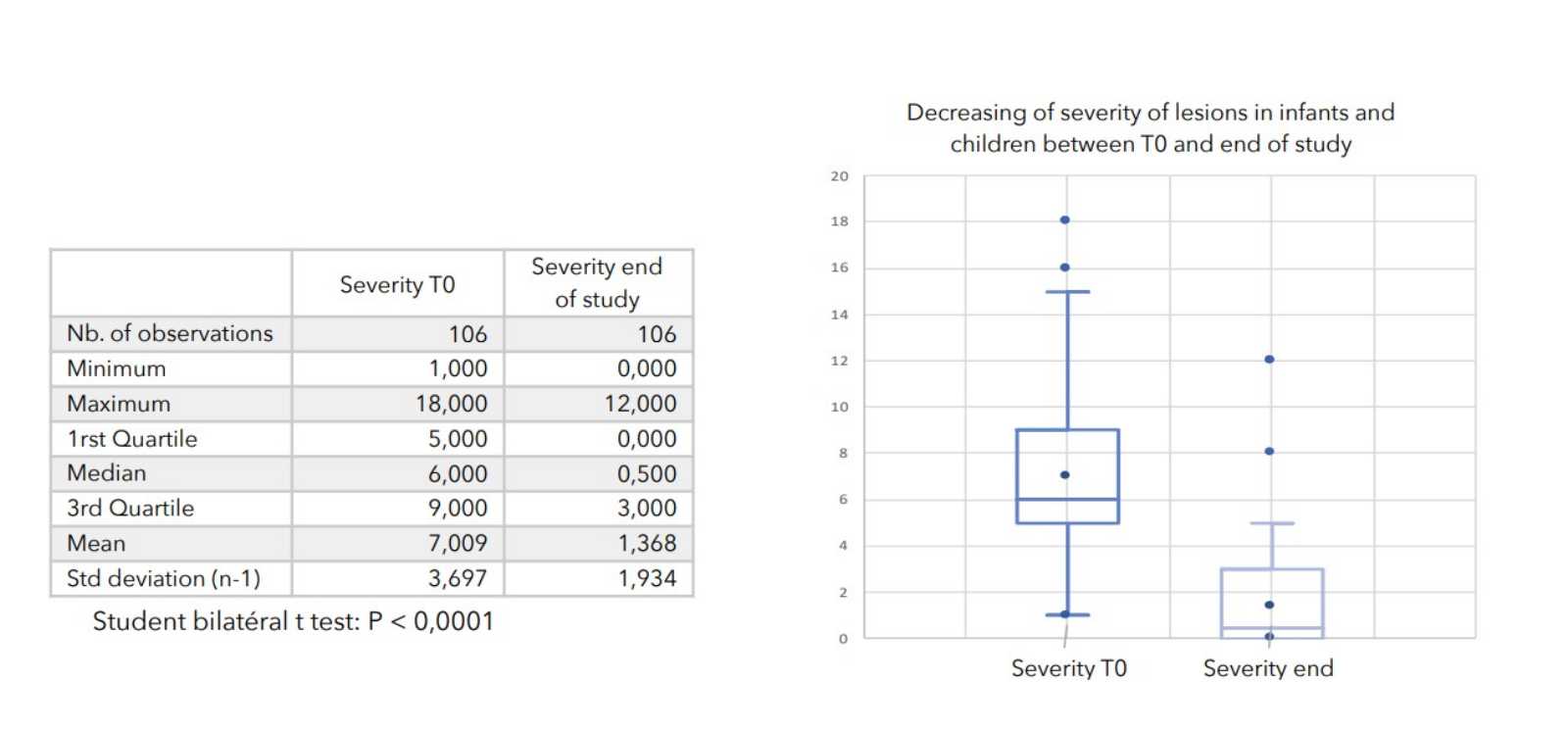
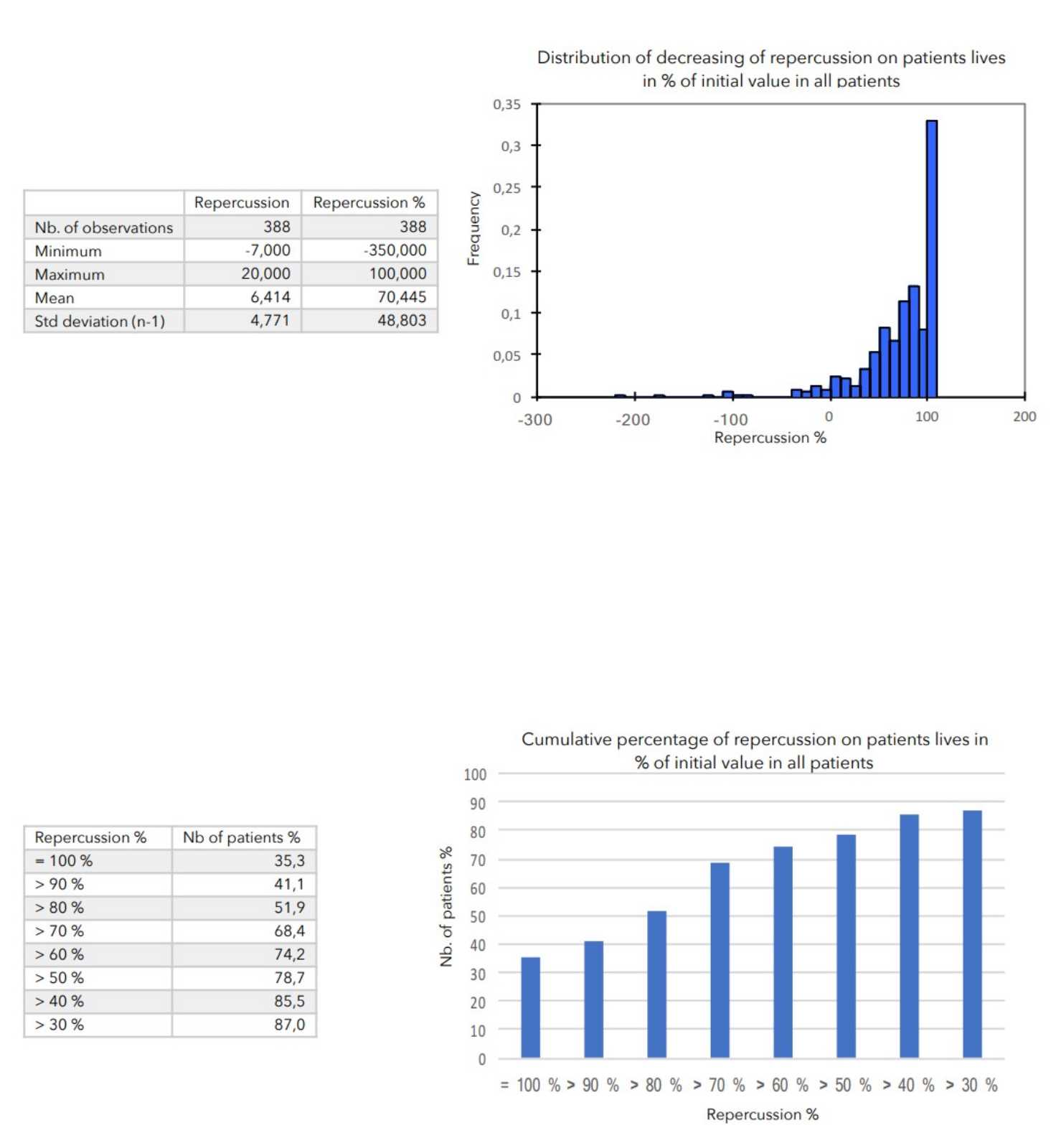
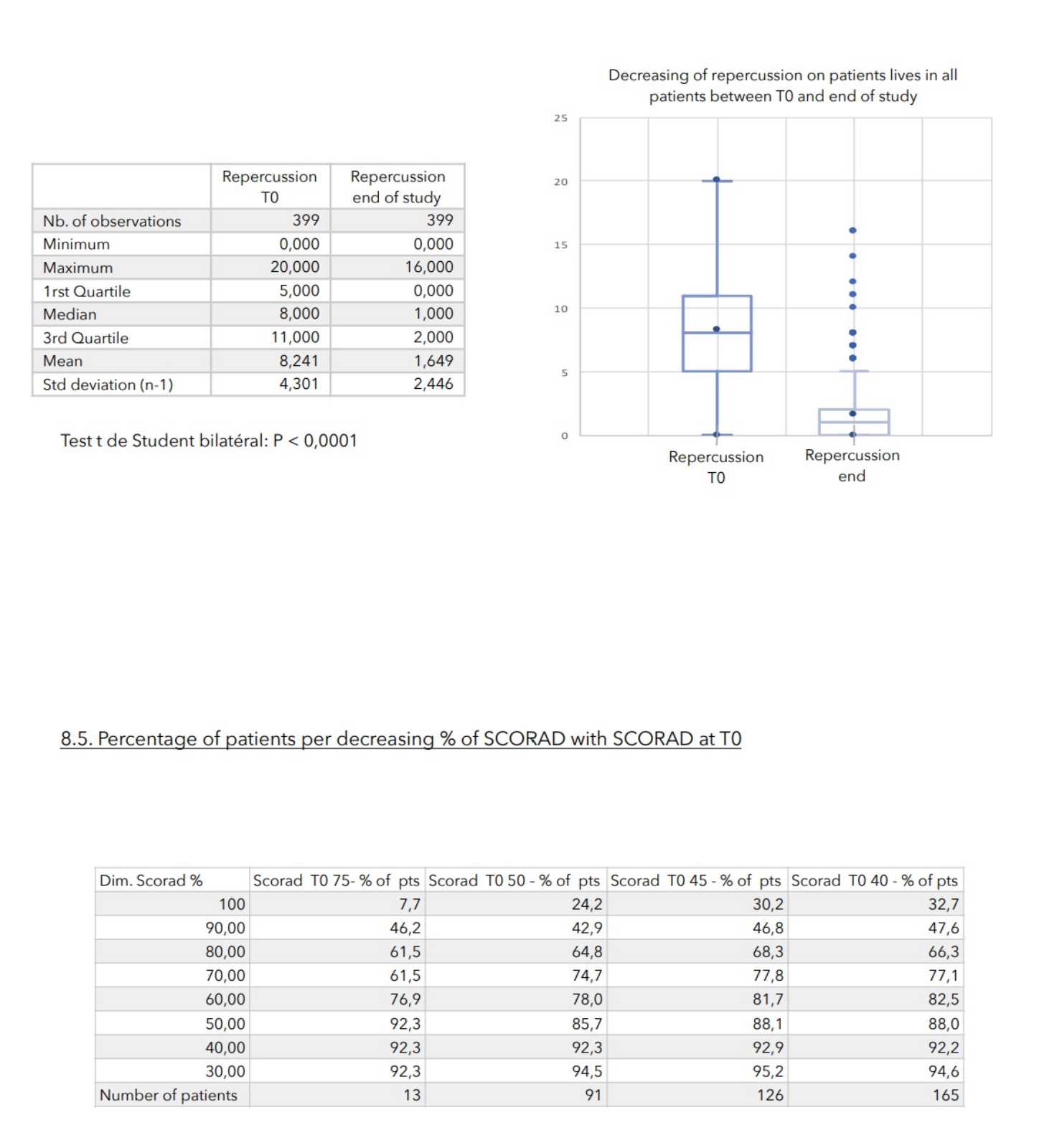
There was a very strong decrease in all the parameters used to assess the severity of atopic dermatitis, with high statical significance as summarized in the table below.
A consensus among specialists in this disease is that a product is effective when it achieves a SCORAD decrease of more than 30%. Thus, Zematopic® eczema therapy body cream has been shown to be effective in more than 90% of cases in children and infants and in approximately 88% of cases in adults.
